U.S. stock futures are down sharply in premarket trading Thursday amid a wider global market selloff as falling crude prices once again take their toll on markets.
Brent crude was down $0.57 to $30.27 per barrel while West Texas crude contracts for March delivery were down $0.98 to $26.47 per barrel.
Asian markets once again led the way down as Hong Kong's Hang Seng closed trading 3.9% lower and Japan's Nikkei fell 2.3%. The Shanghai Composite Index is closed this week in recognition of China's Lunar New Year holiday.
In Europe, France's CAC 40 was falling the hardest, down nearly 4%, while the German DAX and U.K. FTSE 100 were down 2.2% and 2.01%, respectively.
Today, Federal Reserve Chair Janet Yellen continues her testimony before Congress. Yellen began her testimony Wednesday, telling U.S. lawmakers that the central bank recognizes that there are still plenty of obstacles for the U.S. economy to overcome. She did not, however, give any solid indications on what policy tweaks the bank was prepared to make to overcome those headwinds.
The last leg of earnings season continues today. More than 75% of company's scheduled to report their quarterly results have done so already.
Thursday, February 11, 2016
Wednesday, February 10, 2016
Why $CRTO is UP 18% #PreMarketMover ?
Criteo crossed $1 billion in revenue in 2015. Criteo (NASDAQ: CRTO) reported Q4 EPS of EUR0.66, EUR0.26 better than the analyst estimate of EUR0.40. Revenue for the quarter came in at EUR146 million versus the consensus estimate of EUR138.2 million.

SEE ALSO: Wednesday's Scanner

SEE ALSO: Wednesday's Scanner
$AKAM today's scanner #PreMarketMover
Cloud computing company Akamai saw stocks pop after it reported earnings and revenue that beat analysts' expectations. The enterprise software company posted earnings of 72 cents per share on revenue of $579 million, excluding items, better than the 62 cents on $569 million expected by Wall Street.
Akamai Technologies (NASDAQ: AKAM) reported Q4 EPS of $0.72, $0.10 better than the analyst estimate of $0.62. Revenue for the quarter came in at $579 million versus the consensus estimate of $560.72 million.
The Company also announces today that its Board of Directors has authorized a new $1 billion share repurchase program, effective from February 9, 2016 through December 31, 2018. The Company's goal for this program is to offset the dilution created by its employee equity compensation programs and provide the flexibility to increase its capital distributions to shareholders as business and market conditions warrant.

SEE ALSO: Wednesday's Scanner
Akamai Technologies (NASDAQ: AKAM) reported Q4 EPS of $0.72, $0.10 better than the analyst estimate of $0.62. Revenue for the quarter came in at $579 million versus the consensus estimate of $560.72 million.
The Company also announces today that its Board of Directors has authorized a new $1 billion share repurchase program, effective from February 9, 2016 through December 31, 2018. The Company's goal for this program is to offset the dilution created by its employee equity compensation programs and provide the flexibility to increase its capital distributions to shareholders as business and market conditions warrant.

SEE ALSO: Wednesday's Scanner
Monday, February 8, 2016
$AUPH today's scanner Announces Preliminary Topline Data #BeforeBell @ $2.73 UP 25%
Aurinia Pharmaceuticals Inc. (NASDAQ:AUPH / TSX:AUP) (“Aurinia” or the “Company”) announced today that it has completed a preliminary analysis of its AURION (Aurinia early Urinary protein Reduction Predicts Response) study. In the first seven patients that have reached at least eight weeks of therapy in the AURION study, 100% (7/7) have achieved at least a 25% reduction in proteinuria compared to study entry. A 25% reduction in proteinuria has been shown to be predictive of a positive clinical response at 24weeks. All of the other pre-specified eight week biomarkers of active lupus nephritis (LN) have also improved and are trending towards normalization. These biomarkers have also been shown to be predictive of a positive clinical response at 24 weeks.
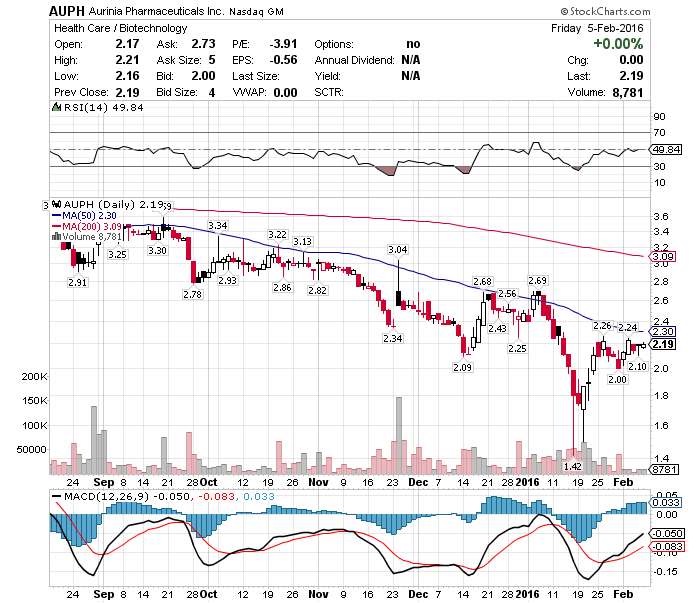
SEE ALSO: Monday's Scanner

SEE ALSO: Monday's Scanner
$SGMO today's scanner on FDA Clearance #PreMarketGainers @ $6.40 UP 13% TP @ $7
RICHMOND, Calif., Feb. 8, 2016 /PRNewswire/ -- Sangamo BioSciences, Inc. (SGMO), the leader in therapeutic genome editing, announced that the U.S. Food and Drug Administration (FDA) has cleared the Company's Investigational New Drug (IND) application for SB-318, a single treatment strategy intended to provide a life-long therapy for Mucopolysaccharidosis Type I (MPS I). The SB-318 IND application is now active and enables Sangamo to initiate a Phase 1/2 clinical study (SB-318-1502) designed to assess the safety, tolerability and potential efficacy of SB-318 in adults with varying severities of MPS I.
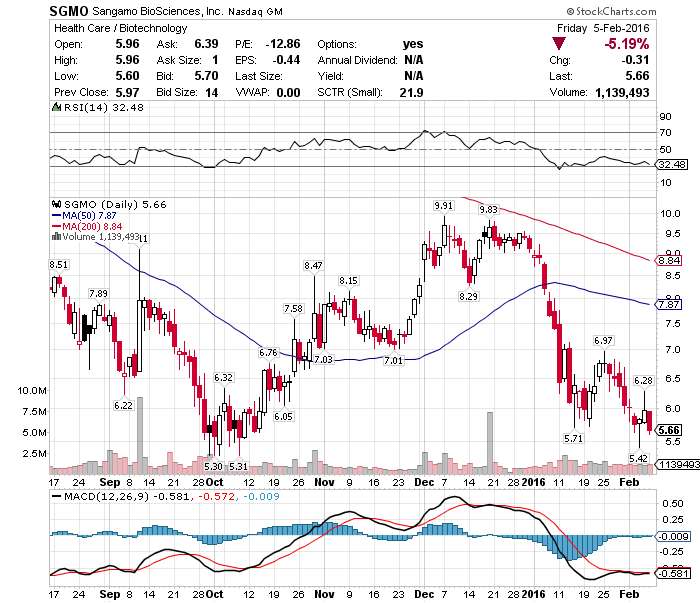
SEE ALSO: Monday's Scanner

SEE ALSO: Monday's Scanner
Ετικέτες
biotech,
fda,
healthcare,
pharmaceuticals,
sgmo
Subscribe to:
Posts (Atom)

